Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: What are Acids and Bases?
Part a: Properties of Acids and Bases
Part a: Properties of Acids and Bases
Part b:
Models of Acids and Bases
The Big Idea:
Acids and bases play a vital role in chemistry, with distinct properties such as taste, feel, reactivity, and interaction with indicators. This lesson explores the characteristic properties of acids and bases, helping students connect real-world observations to chemical behavior.
 Acids and Bases in Chemistry
Acids and Bases in Chemistry
Two of the most common classes of chemical compounds are acids and bases. Acids and bases serve crucial roles in a variety of reactions - in the Chemistry lab, in biological systems, and in industrial processes. They are responsible for adjusting the pH of aqueous solutions, which in turn can affect reaction rates and solubilities of substances. Acids and bases have a predictable behavior and a unique set of properties. They are commonly found in household products and foods. They are deserving of an entire chapter in our Chemistry Tutorial.
Properties of Acids
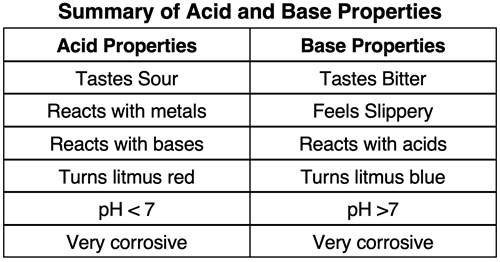
 The word acid comes from the Latin word acidus, which means to be sour. Whether it be the citric acid in a lemon or the lactic acid in soured milk, substances containing acids have a sour taste. Acids are known to react with metals to produce hydrogen gas. And they react with carbonates (e.g. calcium carbonate) to produce carbon dioxide gas. Acidic solutions have a relatively low pH value (less than 7) and turn blue litmus paper red. Common examples of acids you may encounter in a Chemistry lab include sulfuric acid (H2SO4), nitric acid (HNO3), hydrochloric acid (HCl), acetic acid (HC2H3O2), and phosphoric acid (H2SO4). Acids are considered highly corrosive and should be handled with extreme caution. (NOTE: names and formulas of acids were discussed in Chapter 4 of our Chemistry Tutorial.)
The word acid comes from the Latin word acidus, which means to be sour. Whether it be the citric acid in a lemon or the lactic acid in soured milk, substances containing acids have a sour taste. Acids are known to react with metals to produce hydrogen gas. And they react with carbonates (e.g. calcium carbonate) to produce carbon dioxide gas. Acidic solutions have a relatively low pH value (less than 7) and turn blue litmus paper red. Common examples of acids you may encounter in a Chemistry lab include sulfuric acid (H2SO4), nitric acid (HNO3), hydrochloric acid (HCl), acetic acid (HC2H3O2), and phosphoric acid (H2SO4). Acids are considered highly corrosive and should be handled with extreme caution. (NOTE: names and formulas of acids were discussed in Chapter 4 of our Chemistry Tutorial.)
 Properties of Bases
Properties of Bases
Bases are sometimes referred to as alkaline substances. The word alkaline comes from the Arabic word al-qili which means the ashes of saltwort (a plant). Saltwort is a plant that is rich in potassium and sodium. When burned, the ashes consisted of potassium carbonate (potash) and sodium carbonate (soda ash). Both were known to react with and neutralize acidic solutions. As such, the term alkaline came to be used to describe any substance that could neutralize an acid. Today we refer to such alkaline substances as bases.
 Unlike the sour taste of an acid-containing substances, a substance containing a base often taste bitter. Products consisting of bases tend to have a slippery feel; soap is a great example of the slippery feel of bases. Bases turn red litmus paper blue and have a relatively high pH value (greater than 7). Common examples of acids you may encounter in a Chemistry lab include sodium hydroxide (NaOH), potassium hydroxide (KOH), ammonia (NH3), magnesium hydroxide (Mg(OH)2) and calcium hydroxide (Ca(OH)2). Like acids, bases can be very corrosive and should be handled with care.
Unlike the sour taste of an acid-containing substances, a substance containing a base often taste bitter. Products consisting of bases tend to have a slippery feel; soap is a great example of the slippery feel of bases. Bases turn red litmus paper blue and have a relatively high pH value (greater than 7). Common examples of acids you may encounter in a Chemistry lab include sodium hydroxide (NaOH), potassium hydroxide (KOH), ammonia (NH3), magnesium hydroxide (Mg(OH)2) and calcium hydroxide (Ca(OH)2). Like acids, bases can be very corrosive and should be handled with care.
Chemical Reactions Involving Acids and Bases
 The corrosiveness of acids and bases is due to their tendency to readily react. A few reactions are worth mentioning. Acids are known to react with metals to produce hydrogen gas and a salt. A common classroom example includes the reaction of zinc metal with an aqueous solution of hydrochloric acid.
The corrosiveness of acids and bases is due to their tendency to readily react. A few reactions are worth mentioning. Acids are known to react with metals to produce hydrogen gas and a salt. A common classroom example includes the reaction of zinc metal with an aqueous solution of hydrochloric acid.
Zn(s) + 2 HCl(aq) → H2(g) + ZnCl2(aq)
The reaction is a single replacement reaction in which the metal replaces the H+ ion in the acid. This is just one example of several possible acid-metal interactions. Here’s a couple of other common classroom examples:
Mg(s) + 2 HCl(aq) → H2(g) + MgCl2(aq)
Cu(s) + 2 HNO3(aq) → H2(g) + Cu(NO3)2(aq)
 Acids are also known to react with metal carbonates and metal bicarbonates. Metal carbonates include CaCO3, MgCO3, and Na2CO3. Sodium bicarbonate, NaHCO3, is the bicarbonate in baking soda. The reactions typically produce carbon dioxide, water, and a salt. The salt (ionic compound) is formed from the cation of the carbonate and the anion of the acid. Example reactions include:
Acids are also known to react with metal carbonates and metal bicarbonates. Metal carbonates include CaCO3, MgCO3, and Na2CO3. Sodium bicarbonate, NaHCO3, is the bicarbonate in baking soda. The reactions typically produce carbon dioxide, water, and a salt. The salt (ionic compound) is formed from the cation of the carbonate and the anion of the acid. Example reactions include:
CaCO3(s) + H2SO4(aq) → H2O(l) + CO2(g) + CaSO4(aq)
CaCO3(s) + 2 HNO3(aq) → H2O(l) + CO2(g) + Ca(NO3)2(aq)
CaCO3(s) + 2 HCl(aq) → H2O(l) + CO2(g) + CaCl2(aq)
The above reactions are a common cause of the weathering of limestone statues. Calcium carbonate, a major constituent of limestone, will react with acids in rain over the course of several years, resulting in its gradual deterioration.
A common household cleaning hack involves reacting vinegar and baking soda. The sodium bicarbonate (NaHCO3) of baking soda and the acetic acid (HC2H3O2) of vinegar react to produce bubbles of CO2 gas, water, and sodium acetate.
NaHCO3(s) + HC2H3O2(aq) → H2O(l) + CO2(g) + NaC2H3O2(aq)
The fizzing effect which results from the generation of the CO2 gas is often able to dislodge dirt and grime. While it isn’t a powerful cleaning technique, it does have its place in cleaning objects that are not well-suited to exposure to large quantities of water (e.g., the sweat-stained bill of a baseball cap). (And this is where we add: it’s another example of Chemistry for Better Living.) It’s worth noting that the actual reaction that initially occurs involves the production of carbonic acid - H2CO3.
NaHCO3(s) + HC2H3O2(aq) → H2CO3(aq) + NaC2H3O2(aq)
The carbonic acid is relatively unstable and quickly decomposes into carbon dioxide and water which explains the previous equation.
 The above reaction is perhaps the most common reaction of acids and bases. An acid reacts with a base to produce a salt and water. It is referred to as an acid-base neutralization reaction. Another example includes hydrochloric acid reacting with sodium hydroxide to produce sodium chloride and water:
The above reaction is perhaps the most common reaction of acids and bases. An acid reacts with a base to produce a salt and water. It is referred to as an acid-base neutralization reaction. Another example includes hydrochloric acid reacting with sodium hydroxide to produce sodium chloride and water:
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Other examples include:
2 HCl(aq) + Mg(OH)2(aq) → MgCl2(aq) + 2 H2O(l)
H2SO4(aq) + 2 NaOH(aq) → Na2SO4(aq) + 2 H2O(l)
In all three cases above, the acid and the base are written as reactants on the left side of the reaction symbol. The products are a salt (ionic compound) and water. We will study acid-base neutralization reactions in more detail in Lesson 4 of this chapter.
Litmus Paper and pH Indicators in Chemistry
As mentioned above, aqueous solutions of acids have a relatively low pH (less than 7) and bases have a relatively high pH (greater than 7). The pH of an aqueous solution can be determined using a pH meter or an acid-base indicator. An indicator is a substance that will have a different color in an acidic solution than in a basic solution. The two most common indicators used in Chemistry class are litmus paper and phenolphthalein.
 Litmus paper is a thin strip of filter paper than contains litmus. Litmus is a mixture of natural dyes that exhibit a red color in acidic solutions and a blue color in basic solutions. The dyes are soluble in water and can be infused onto absorbent paper and cut into strips. If the paper and strips are manufactured in an acidic environment, red litmus paper results. Blue litmus paper is manufactured by using an alkaline environment during processing. When a strip of red litmus paper is dipped in a basic solution, the paper turns blue. When a blue strip of litmus paper is dipped in an acidic solution, it turns red.
Litmus paper is a thin strip of filter paper than contains litmus. Litmus is a mixture of natural dyes that exhibit a red color in acidic solutions and a blue color in basic solutions. The dyes are soluble in water and can be infused onto absorbent paper and cut into strips. If the paper and strips are manufactured in an acidic environment, red litmus paper results. Blue litmus paper is manufactured by using an alkaline environment during processing. When a strip of red litmus paper is dipped in a basic solution, the paper turns blue. When a blue strip of litmus paper is dipped in an acidic solution, it turns red.

Phenolphthalein, often abbreviated as phth, is a compound used as an acid-base indicator. It is purchased as a fine powder and dissolved in very small quantities in an alcohol solution. A couple of drops of the phth solution can be added to test the acidity of an unknown solution. The drops of phth will turn pink to a deep magenta in basic solutions and be colorless in acidic solutions. Phenolphthalein is commonly used in Chemistry labs as a pH indicator for titration experiments. Titrations will be discussed in Lesson 4. The Chemistry of pH and acid-base indicators will be detailed in Lesson 2.
Everyday Examples of Acids and Bases
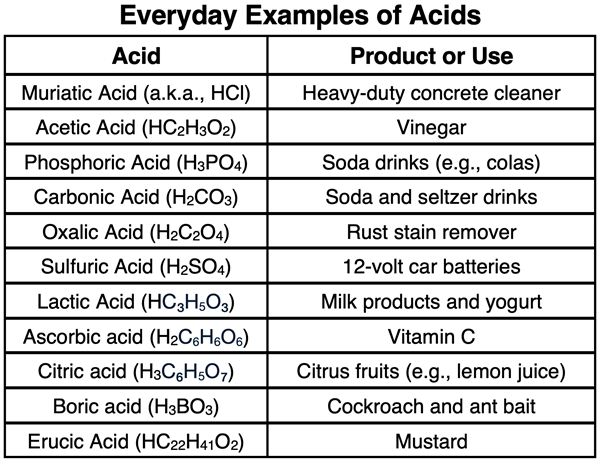
Students are often amazed by how prevalent acids and bases are in their everyday lives. Examples of several common acids and their uses are shown in the table below:
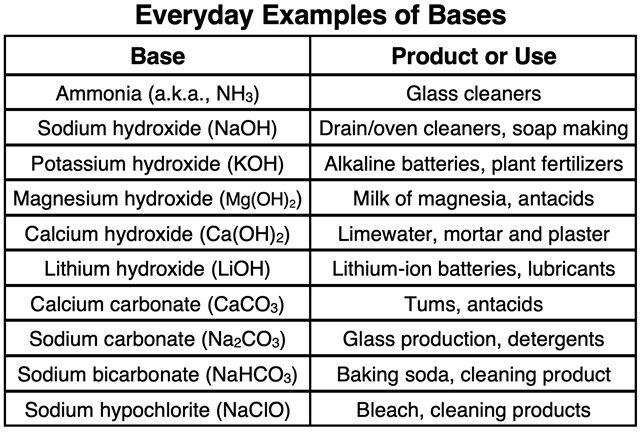
Examples of several common bases and their uses include:
As we proceed through this chapter, we will learn more details about many of the terms (pH, indicators, neutralization, etc.) introduced on this page. On the next page of Lesson 1, we will learn about what makes a chemical compound an acid or a base. Before you leave, take some time to implement one or more of the practice opportunities suggested below.
Before You Leave - Practice and Reinforcement
Now that you've done the reading, take some time to strengthen your understanding and to put the ideas into practice. Here's some suggestions.
- Try the Apprentice Difficulty Level of the Acid-Base Properties Concept Builder. The Master and Wizard level will be more suitable after a couple more lessons.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on Properties of Acids and Bases. Save it to a safe location and use it as a review tool.
Check Your Understanding of Acid and Base Properties
Use the following questions to assess your understanding of the differences and similarities between acids and bases. Tap the Check Answer buttons when ready.
1. Which of the followinig properties are characteristic of acids only, bases only, and of both acids and bases?
- Has a pH value.
- Reacts with metals to produce H2 gas.
- Has a sour taste.
- Turns pink when two drops of phenolpthalein are stirred into it.
- Turns red litmus paper blue.
- Is corrosive
- Has a pH value of 2.5.
- Feels slippery.
- Reacts with acids.
- Has a bitter taste.
- Reacts with carbonates to produce CO2.
- Has a pH value of 12.3.
2. The lesson mentions that acids turn blue litmus paper red. What color does an acid turn red litmus paper?
3. Review all the acid formulae mentioned on this page (the table near the end of the page would be a good starting point). What do all the formulae have in common?
4. Like acid formulae, many (but not all) of the formulae of base have one thing in common. What do all the formulae have in common?